New Delhi: As part of COVID-19 mitigation mission of Country, CSIR has strategized its R&D to develop, integrate, scale-up, and deploy necessary technological interventions for combating Coronavirus pandemic in the country. Considering the multifarious problems created by coronavirus, which require interventions, the CSIR under the guidance of its Director General, Dr. Shekhar Mande has formed five verticals to coordinate various research activities into Digital and Molecular Surveillance, Drugs & Vaccines, Rapid and Economical Diagnostics, Hospital Assistive Devices & PPEs, and Supply Chain and Logistics.
Since testing is vital component in COIVD-19 mitigation, CSIR-IIIM, Jammu a constituent laboratory of CSIR has partnered with Reliance Industries Limited (RIL) to develop and scale-up a new Reverse Transcriptase-Loop Mediated Isothermal Amplification (RT-LAMP) based COVID-19 diagnostic kit for which a formal MOU has also been signed between CSIR-IIIM, Jammu and RIL.

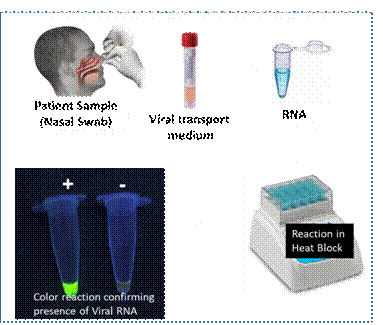
COVID-19 RT-LAMP test is a nucleic acid based test carried out from nasal/throat swab sample from patients. The test recipe has been developed and successfully demonstrated using synthetic templates. It is rapid (45-60 min), cost effective and accurate test. It has been tested with a small number of patients samples and validating the kit on more number of patient samples is planned and will be done together with RIL.
The advantage of this test is that the RT-LAMP based COVID-19 kit components are easily available and these can be completely manufactured in India. While the, the current COVID-19 testing is done by real-time PCR their components are mostly imported. Further these tests are expensive; require highly trained manpower, costly instruments and a relatively high-end lab and cannot be deployed at remote locations in quarantine centers, airports and railway stations.
On the other hand, the RT-LAMP test can be done in a single tube with minimal expertise in a very basic lab setup like mobile units / kiosks for testing at Airports, Railway Stations, Bus Stands and other public places. The end detection of the test is a simple colored reaction, which is easily visible in UV light, and now is being modified such that it can be detected in regular light.
After testing the accuracy of the kit, on a much larger number of patients, CSIR-IIIM and RIL will jointly approach ICMR for approval. RIL plans to rapidly scale up the tests for the larger population and use it for easy, rapid and widespread diagnosis for COVID-19 detection for the larger interest of society.





